
Chemistry, 05.05.2020 10:11 ashleytellez
From this combustion equation, 2CH22 + 3102 - 22H,0 + 2000, calculate the liters of
carbon dioxide produced when 16.9 grams of CH are combusted

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2


Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3
You know the right answer?
From this combustion equation, 2CH22 + 3102 - 22H,0 + 2000, calculate the liters of
carbon dio...
carbon dio...
Questions


English, 15.12.2020 02:00

Mathematics, 15.12.2020 02:00



Physics, 15.12.2020 02:00





Mathematics, 15.12.2020 02:00


Mathematics, 15.12.2020 02:00

Mathematics, 15.12.2020 02:00

Mathematics, 15.12.2020 02:00

Mathematics, 15.12.2020 02:00

English, 15.12.2020 02:00



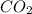 produced are, 26.7 liters.
produced are, 26.7 liters.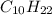
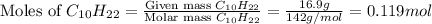
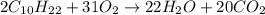
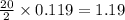 moles of
moles of 


