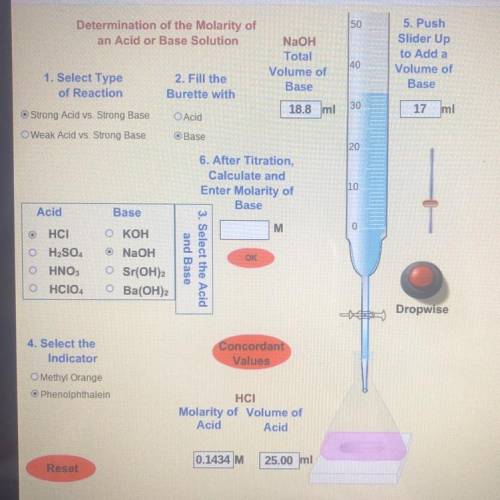
Find Number 6. After Titration, Calculate and Enter Molarity of Base
To calculate the mo...

Find Number 6. After Titration, Calculate and Enter Molarity of Base
To calculate the molarity of your base, you will first need to write out the balanced equation for the reaction between your acid and base . You will then use the Molarity of Acid , Volume of Acid, and Volume of Base Used to determine the Molarity of the Base. Your sig figs should match with the sig figs used in the Molarity of the Acid.
I’ll give Brainliest

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3
You know the right answer?
Questions










Biology, 31.12.2021 19:30




English, 31.12.2021 19:30



Mathematics, 31.12.2021 19:30

Chemistry, 31.12.2021 19:30

Social Studies, 31.12.2021 19:30



