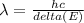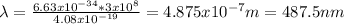Chemistry, 05.05.2020 13:00 kraigstlistt
Hydrogen atoms absorb energy so that the electrons are excited to n=4. Calculate the wavelength, in
nm, of the photon emitted when the electron relaxes to n=2.
487nm
O 456nm
4.56 x 10 nm
4.87 x 107nm

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

You know the right answer?
Hydrogen atoms absorb energy so that the electrons are excited to n=4. Calculate the wavelength, in<...
Questions



History, 15.01.2021 21:40

Social Studies, 15.01.2021 21:40

Mathematics, 15.01.2021 21:40




Mathematics, 15.01.2021 21:40

Mathematics, 15.01.2021 21:40

Geography, 15.01.2021 21:40


Mathematics, 15.01.2021 21:40


English, 15.01.2021 21:40



Health, 15.01.2021 21:40


French, 15.01.2021 21:40

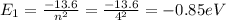 (excited)
(excited)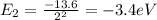 (relaxes)
(relaxes)