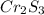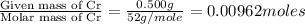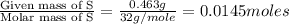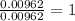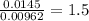Chemistry, 05.05.2020 15:08 teionamwhite2262
A 0.500-g sample of chromium metal reacted with sulfur powder to give 0.963 g of product. Calculate the empirical formula of the chromium sulfide.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1


Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3
You know the right answer?
A 0.500-g sample of chromium metal reacted with sulfur powder to give 0.963 g of product. Calculate...
Questions

Mathematics, 28.09.2021 23:40



Mathematics, 28.09.2021 23:40

Mathematics, 28.09.2021 23:40










Mathematics, 28.09.2021 23:40




Mathematics, 28.09.2021 23:40

Mathematics, 28.09.2021 23:40