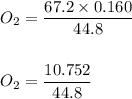Chemistry, 05.05.2020 15:06 rmans22209
Assuming that pressure and temperature are constant, how many liters of oxygen gas will be required to produce 0.160 L of nitrogen gas.
4 NH3 (g) + 3 O2 (g) → 6 H2O (g) + 2 N2 (g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
Assuming that pressure and temperature are constant, how many liters of oxygen gas will be required...
Questions





English, 06.04.2021 01:00


Mathematics, 06.04.2021 01:00


History, 06.04.2021 01:00



Mathematics, 06.04.2021 01:00

Computers and Technology, 06.04.2021 01:00




Mathematics, 06.04.2021 01:00


Mathematics, 06.04.2021 01:00