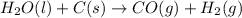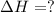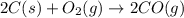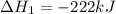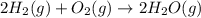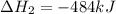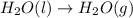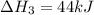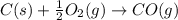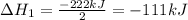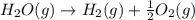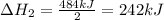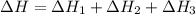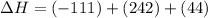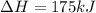Chemistry, 05.05.2020 16:29 shawn20034
2C(s)+O2(g)2H2(g)+O2(g)H2O(l)→2CO(g )→2H2O(g)→H2O(g)ΔHΔHΔH=−222kJ=−484k J=+44kJ Use the thermochemical data above to calculate the change in enthalpy for the reaction below. H2O(l)+C(s)→CO(g)+H2(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

You know the right answer?
2C(s)+O2(g)2H2(g)+O2(g)H2O(l)→2CO(g )→2H2O(g)→H2O(g)ΔHΔHΔH=−222kJ=−484k J=+44kJ Use the thermochemic...
Questions

English, 14.11.2019 13:31

Geography, 14.11.2019 13:31


Mathematics, 14.11.2019 13:31


Mathematics, 14.11.2019 13:31


History, 14.11.2019 13:31


Mathematics, 14.11.2019 13:31


Mathematics, 14.11.2019 13:31


English, 14.11.2019 13:31


Biology, 14.11.2019 13:31


Computers and Technology, 14.11.2019 13:31

Chemistry, 14.11.2019 13:31