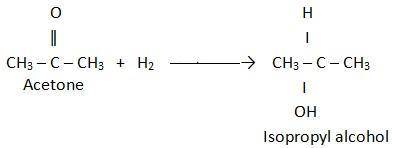
Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon–oxygen double bond. calculate the enthalpy of reaction using the bond energies given. bond: c=o h–h c–h o–h c–c c–o bond energy (kj/mol): 745 432 413 467 347 358 multiple choice –61 kj +61 kj –366 kj –484 kj +366 kj

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:20
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

You know the right answer?
Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon–oxygen do...
Questions





Social Studies, 22.03.2021 16:20



Mathematics, 22.03.2021 16:20



Advanced Placement (AP), 22.03.2021 16:20




English, 22.03.2021 16:20





World Languages, 22.03.2021 16:20