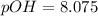Chemistry, 05.05.2020 16:09 alexabrandon1848
A 25.00-mL solution of 0.1500 M methylamine (CH3NH2) is titrated with a standardized 0.1025 M solution of HCl at 25°C. Enter your numbers to 2 decimal places. Kb = 4.4x10-4 What is the pH of the methylamine solution before titrant is added? 11.91 How many milliliters of titrant are required to reach the equivalence point? 36.59 What is the pH at the equivalence point?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1


Chemistry, 23.06.2019 09:00
How many moles of sulfur dioxide are in 2.26 × 10^33 sulfur dioxide molecules?
Answers: 3

Chemistry, 23.06.2019 10:00
Why sncl2 is solid while sncl4 is liquid at room temprature explain it in easy way
Answers: 1
You know the right answer?
A 25.00-mL solution of 0.1500 M methylamine (CH3NH2) is titrated with a standardized 0.1025 M soluti...
Questions

English, 12.12.2020 16:50

Advanced Placement (AP), 12.12.2020 16:50

Social Studies, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50

Social Studies, 12.12.2020 16:50


English, 12.12.2020 16:50



Mathematics, 12.12.2020 16:50

History, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50

Business, 12.12.2020 16:50

English, 12.12.2020 16:50

Mathematics, 12.12.2020 16:50


History, 12.12.2020 16:50


![pOH = \frac{1}{2}[pK_b \ - \ log \ C]](/tpl/images/0640/0162/873d4.png)
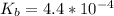
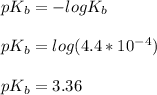
![pOH = \frac{1}{2}[3.36\ - \ log \0.15]](/tpl/images/0640/0162/71cf9.png)
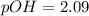
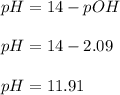
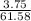
![pOH = \frac{1}{2}[pK_w+pK_b+log \ C]](/tpl/images/0640/0162/af00e.png)
![pOH = \frac{1}{2}[14+3.36+log \ 0.061]](/tpl/images/0640/0162/443df.png)
![pOH = \frac{1}{2}[16.15]](/tpl/images/0640/0162/bf604.png)