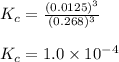Chemistry, 05.05.2020 17:34 shamim5364
Iron (III) oxide and hydrogen react to form iron and water, like this: Fe 03(s)+3H9)2Fe(s)+3HO) At a certain temperature, a chemist finds that a 8.9 L reaction vessel containing a mixture of iron(III) oxide, hydrogen, Iron, and water at equilbrium has the following composition compound amount Fe 3.95 g H 4.77 g Fe 4.38 g H2 2.00 g Calculate the value of the equilibrium constant Kc for this reaction. Round your answer to 2 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Why are gamma rays not affected by a magnet as they pass over it? gamma rays are composed of only energy. gamma rays do not have enough mass to be affected. gamma rays do not have the right electrical charge to be affected. gamma rays move too fast for anything to affect their pathway.
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1
You know the right answer?
Iron (III) oxide and hydrogen react to form iron and water, like this: Fe 03(s)+3H9)2Fe(s)+3HO) At a...
Questions

Mathematics, 21.09.2020 14:01

Mathematics, 21.09.2020 14:01


Health, 21.09.2020 14:01




Mathematics, 21.09.2020 14:01

History, 21.09.2020 14:01



Mathematics, 21.09.2020 14:01





Computers and Technology, 21.09.2020 14:01

Computers and Technology, 21.09.2020 14:01


Advanced Placement (AP), 21.09.2020 14:01

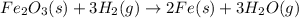
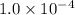
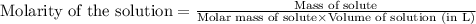
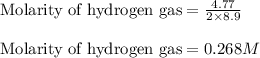
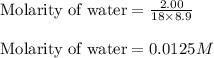
![K_{eq}=\frac{[H_2O]^3}{[H_2]^3}](/tpl/images/0640/8214/066de.png)