
Chemistry, 05.05.2020 17:07 Natavia3402
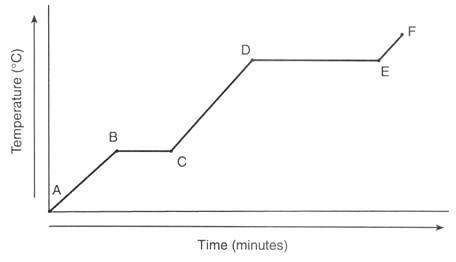
A 47.7 g chunck of ice at -58.0 °C is heated until it completely melts. Find the total amount of heat in joules for this process to occur. = 2.09J/g°C
= 2.03 J/g°C
= 4.184 J/g°C
∆ = 334 J/g
∆ = 2260 J/g
What is the total amount of energy needed to overall kilojoules (three significant figures)?
A. 21.7 kJ
B. 5.78 kJ
C. 15.9 kJ
D. 10.1

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 10:10
Solid tin exists in two forms: white and gray. for the transformation sn(s, white) → sn(s, gray) the enthalpy change is -2.1 kj/mol and the entropy change is -7.4 j/(mol*k). a. calculate the gibbs free energy change for the conversion of 1.00 mol white tin to gray tin at -30℃. b. will white tin convert spontaneously to gray tin at -30℃? c. at what temperature are white and gray tin thermodynamically equivalent at a pressure of 1 atm?
Answers: 3

Chemistry, 23.06.2019 11:30
All of the following describe uses of nonrenewable energy sources except
Answers: 3
You know the right answer?
A 47.7 g chunck of ice at -58.0 °C is heated until it completely melts. Find the total amount of hea...
Questions





Biology, 05.08.2019 02:10




Spanish, 05.08.2019 02:10


Mathematics, 05.08.2019 02:10


Mathematics, 05.08.2019 02:10


Mathematics, 05.08.2019 02:10


Mathematics, 05.08.2019 02:10


Mathematics, 05.08.2019 02:10



