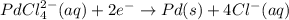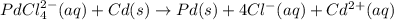Chemistry, 05.05.2020 18:31 cdradlet2001
A voltaic cell that uses the reaction PdCl42−(aq)+Cd(s) → Pd(s)+4Cl−(aq)+Cd2+(aq) has a measured standard cell potential of +1.03 V. You may want to reference (Pages 860 - 867) Section 20.4 while completing this problem. Part A Write the half-cell reaction at the cathode.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
A voltaic cell that uses the reaction PdCl42−(aq)+Cd(s) → Pd(s)+4Cl−(aq)+Cd2+(aq) has a measured sta...
Questions


Biology, 06.12.2021 14:00

Mathematics, 06.12.2021 14:00

English, 06.12.2021 14:00

English, 06.12.2021 14:00

Mathematics, 06.12.2021 14:00




English, 06.12.2021 14:00

Physics, 06.12.2021 14:00



World Languages, 06.12.2021 14:00



History, 06.12.2021 14:00



Biology, 06.12.2021 14:00