
Chemistry, 05.05.2020 18:18 mshepherdmiller
An aqueous solution has [C6H5COOH] = 0.110 M and [Ca(C6H5COO)2] = 0.200 M. Ka = 6.3 × 10-5 for C6H5COOH. The solution volume is 5.00 L. What is the pH of the solution after 10.00 mL of 5.00 M NaOH is added? Group of answer choices 4.81 4.86 4.75 4.70 4.65

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
An aqueous solution has [C6H5COOH] = 0.110 M and [Ca(C6H5COO)2] = 0.200 M. Ka = 6.3 × 10-5 for C6H5C...
Questions



Mathematics, 17.11.2020 21:10

Chemistry, 17.11.2020 21:10

Mathematics, 17.11.2020 21:10

History, 17.11.2020 21:10


English, 17.11.2020 21:10



Mathematics, 17.11.2020 21:10




Advanced Placement (AP), 17.11.2020 21:10


Mathematics, 17.11.2020 21:10



Mathematics, 17.11.2020 21:10

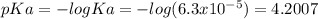
![pH=pKa+log\frac{C6H5COO]}{[C6H5COOH]} =4.2007+log\frac{0.4}{0.11} =4.7614](/tpl/images/0641/1671/7b407.png)
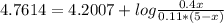
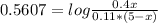
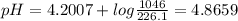
![An aqueous solution has [C6H5COOH] = 0.110 M and [Ca(C6H5COO)2] = 0.200 M. Ka = 6.3 × 10-5 for C6H5C](/tpl/images/0641/1671/b03ac.jpg)


