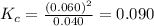Chemistry, 05.05.2020 18:04 AgentPangolin
At high temperatures, carbon reacts with O2 to produce CO as follows: C(s) O2(g) 2CO(g). When 0.350 mol of O2 and excess carbon were placed in a 5.00-L container and heated, the equilibrium concentration of CO was found to be 0.060 M. What is the equilibrium constant, Kc, for this reaction

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1


Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
At high temperatures, carbon reacts with O2 to produce CO as follows: C(s) O2(g) 2CO(g). When 0.350...
Questions



Mathematics, 29.12.2020 22:10


Mathematics, 29.12.2020 22:10



History, 29.12.2020 22:20



Advanced Placement (AP), 29.12.2020 22:20




Mathematics, 29.12.2020 22:20




Mathematics, 29.12.2020 22:20

Social Studies, 29.12.2020 22:20

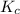 is 0.090.
is 0.090.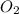 =
= 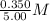 = 0.0700 M
= 0.0700 M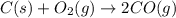
![K_{c}=\frac{[CO]^{2}}{[O_{2}]}](/tpl/images/0641/0273/4df57.png) , where [CO] and
, where [CO] and ![[O_{2}]](/tpl/images/0641/0273/9a638.png) represents equilibrium concentration of CO and
represents equilibrium concentration of CO and ![[CO]=2x=0.060](/tpl/images/0641/0273/522d6.png)