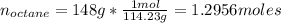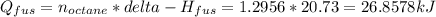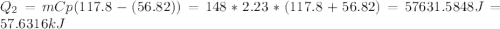Chemistry, 05.05.2020 19:12 alisonlebron15
Calculate the amount of heat needed to melt 148. g of solid octane (C8H18 ) and bring it to a temperature of 117.8 degrees c. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1


Chemistry, 23.06.2019 11:30
Which of the following is the most likeley example of an favorable mutation a. a mutation that makes a rabbit able run faster b. a mutation that changes the rabbit's fur to bright orange c. a mutation that changes the color of the rabbit's eyes d. a mutation that gives a rabbit a third ear
Answers: 1
You know the right answer?
Calculate the amount of heat needed to melt 148. g of solid octane (C8H18 ) and bring it to a temper...
Questions

Chemistry, 20.01.2022 14:00








Computers and Technology, 20.01.2022 14:00

Physics, 20.01.2022 14:00


Mathematics, 20.01.2022 14:00

Mathematics, 20.01.2022 14:00



Chemistry, 20.01.2022 14:00

Computers and Technology, 20.01.2022 14:00

Mathematics, 20.01.2022 14:00