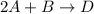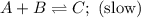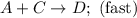Chemistry, 05.05.2020 20:16 jacksonyodell8601
Consider the mechanism. Step 1: A + B ⟶ C A+B⟶C slow Step 2: A + C ⟶ D A+C⟶D fast Overall: 2 A + B ⟶ D 2A+B⟶D Determine the rate law for the overall reaction, where the overall rate constant is represented as k .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1
You know the right answer?
Consider the mechanism. Step 1: A + B ⟶ C A+B⟶C slow Step 2: A + C ⟶ D A+C⟶D fast Overall: 2 A + B ⟶...
Questions

History, 04.10.2019 22:40



Chemistry, 04.10.2019 22:40






Mathematics, 04.10.2019 22:40




Mathematics, 04.10.2019 22:40

History, 04.10.2019 22:40



Business, 04.10.2019 22:40

History, 04.10.2019 22:40

Mathematics, 04.10.2019 22:40

![\text{Rate}=k[A]^2[B]](/tpl/images/0642/1478/15407.png)