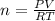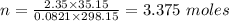Chemistry, 05.05.2020 21:21 janiyahmcgolley
Methane burns in the presence of oxygen to produce carbon dioxide and water in the following reaction:
CH4 (s) + 2 O2 (g) --> CO2 (g) + 2 H2O (l)
What mass of methane (in grams) will require 35.15 L of oxygen to fully react? The pressure is 2.35 atm and the temperature is 15 °C. (R = 0.0821 L atm/mol K)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
Methane burns in the presence of oxygen to produce carbon dioxide and water in the following reactio...
Questions

Mathematics, 09.01.2021 14:00

History, 09.01.2021 14:00

Mathematics, 09.01.2021 14:00


Biology, 09.01.2021 14:00


English, 09.01.2021 14:00

English, 09.01.2021 14:00

SAT, 09.01.2021 14:00

Mathematics, 09.01.2021 14:00

Mathematics, 09.01.2021 14:00

Mathematics, 09.01.2021 14:00

History, 09.01.2021 14:00


Mathematics, 09.01.2021 14:00


Geography, 09.01.2021 14:00

Mathematics, 09.01.2021 14:00

Mathematics, 09.01.2021 14:00