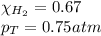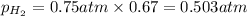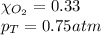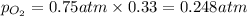Chemistry, 05.05.2020 22:21 ayoismeisjjjjuan
Solve the following, show all work and units for your calculation: Let's say we have a mixture of hydrogen gas
(H2), and oxygen gas (O2). The mixture contains 6.7 mol hydrogen gas and 3.3 mol oxygen gas. The mixture is
in a 300 L container at 273 K and the total pressure of the gas mixture is 0.75 atm. What is the partial
pressure for each gas?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
You know the right answer?
Solve the following, show all work and units for your calculation: Let's say we have a mixture of hy...
Questions





English, 29.07.2019 13:30


Mathematics, 29.07.2019 13:30


Physics, 29.07.2019 13:30



History, 29.07.2019 13:30






Mathematics, 29.07.2019 13:30

History, 29.07.2019 13:30

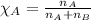
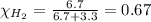
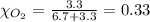
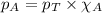
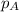 = partial pressure of substance
= partial pressure of substance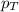 = total pressure
= total pressure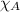 = mole fraction of substance
= mole fraction of substance