

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
For the reaction shown, calculate how many moles of NH3 form when 16.72 moles of reactant completely...
Questions








Mathematics, 08.04.2020 02:14



Mathematics, 08.04.2020 02:14


Social Studies, 08.04.2020 02:14




Arts, 08.04.2020 02:14


Mathematics, 08.04.2020 02:14

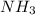 formed are, 22.3 moles.
formed are, 22.3 moles.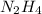 = 16.72 mol
= 16.72 mol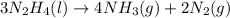
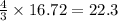 moles of
moles of 

