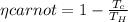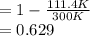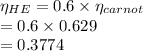Chemistry, 05.05.2020 22:09 aidananderson
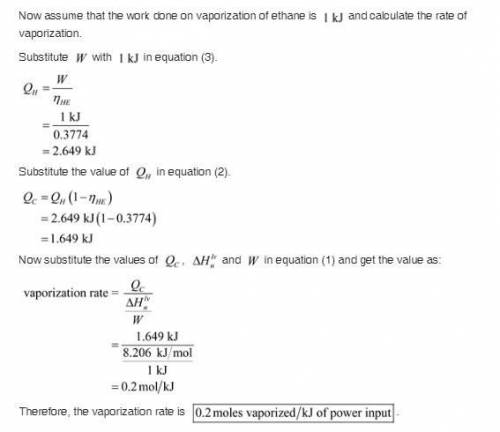
Liquefied natural gas (LNG) is transported in very large tankers, stored as liquid in equilibrium with its vapor at approximately atmospheric pressure. If LNG is essentially pure methane, the storage temperature then is about 111.4 K, the normal boiling point of methane. The enormous amount of cold liquid can in principle serve as a heat sink for an onboard heat engine. Energy discarded to the LNG serves for its vaporization. If the heat source is ambient air at 300 K, and if the efficiency of a heat engine is 61% of its Carnot value, estimate the vaporization rate in moles vaporized per kJ of power output. For methane,

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1


Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1
You know the right answer?
Liquefied natural gas (LNG) is transported in very large tankers, stored as liquid in equilibrium wi...
Questions




Mathematics, 16.05.2021 06:50

Health, 16.05.2021 06:50






Mathematics, 16.05.2021 06:50




Biology, 16.05.2021 06:50




Mathematics, 16.05.2021 06:50

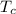 . The heat source to vapourization of methane is ambient air which is at 300 K.
. The heat source to vapourization of methane is ambient air which is at 300 K. 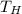
![Vaporization rate = \frac{Q_c}{[\frac{\delta H_n^{lv}}{W}]} ..........(1)](/tpl/images/0643/0740/c73f9.png)
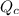 is the heat at temperature
is the heat at temperature 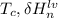 is the phase transition enthalpy of methane and W is the work
is the phase transition enthalpy of methane and W is the work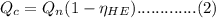
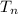 and
and 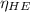 is the efficiency of heat engine
is the efficiency of heat engine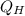 from the relation shown below:
from the relation shown below: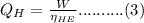
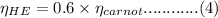
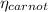 is the carnot engine efficiency
is the carnot engine efficiency