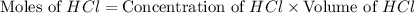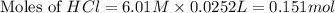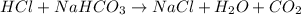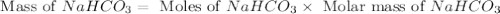Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2



Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
You know the right answer?
Baking soda (sodium bicarbonate) is often used to neutralize spills of acids on the benchtop of the...
Questions

Mathematics, 07.11.2020 01:00








Chemistry, 07.11.2020 01:00

Mathematics, 07.11.2020 01:00

Mathematics, 07.11.2020 01:00

Mathematics, 07.11.2020 01:00

Biology, 07.11.2020 01:00

Biology, 07.11.2020 01:00




Biology, 07.11.2020 01:00

Mathematics, 07.11.2020 01:00

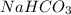 needed is, 12.7 grams.
needed is, 12.7 grams.