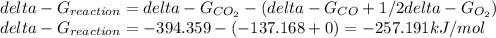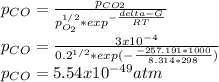Chemistry, 06.05.2020 00:21 Jenniferojeda2002
Hat is the pressure of CO(g) in equilibrium with the CO2(g) and O2(g) in the atmosphere at 25 C? The partial pressure of O2(g) is 0.2 bar and the partial pressure of CO2(g) is 3 * 10-4 bar. CO is extremely poisonous because it forms a very strong complex with hemoglobin. Should you worry?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

You know the right answer?
Hat is the pressure of CO(g) in equilibrium with the CO2(g) and O2(g) in the atmosphere at 25 C? The...
Questions












Mathematics, 09.07.2020 07:01

Biology, 09.07.2020 07:01





Mathematics, 09.07.2020 07:01


History, 09.07.2020 07:01