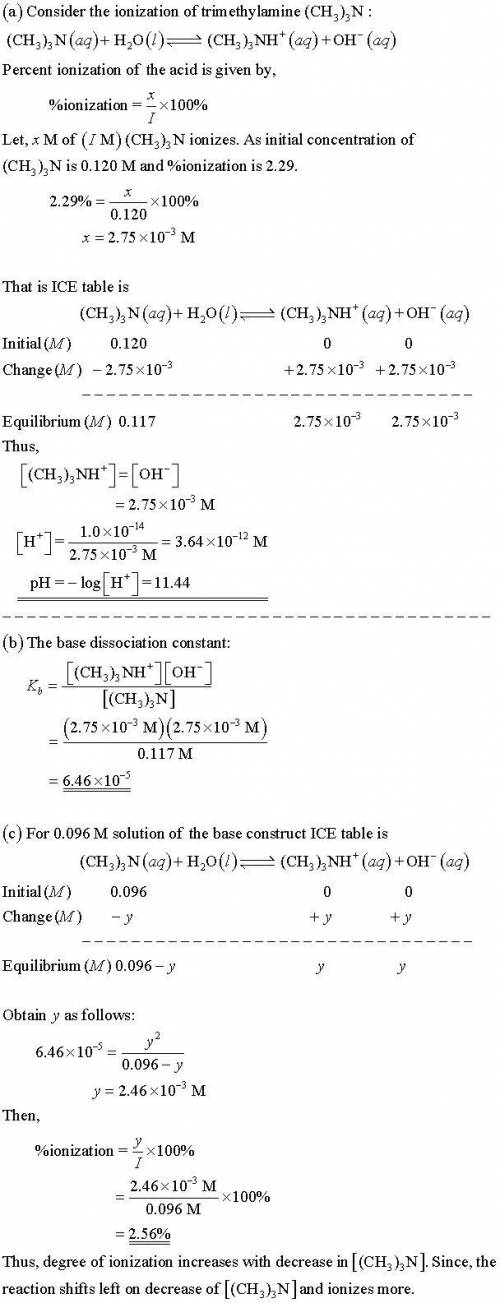
Trimethylamine, (CH3)3N, is a weak base that ionizes in aqueous solution:
(CH3)3N(aq) + H2O(l)...

Chemistry, 06.05.2020 00:18 aliciapinto13
Trimethylamine, (CH3)3N, is a weak base that ionizes in aqueous solution:
(CH3)3N(aq) + H2O(l) (CH3)3NH+(aq) + OH−(aq)
A 0.120 M solution of (CH3)3N(aq) is 2.29% ionized at 25OC.
(a) Calculate [OH− ], [(CH3)3NH+ ], [H3O+ ] and the pH for a 0.120 M (CH3)3N(aq)
solution at 25oC.
(b) Calculate Kb for (CH3)3N at 25oC.
(c) Calculate the degree of ionization, α, of a 0.096 M solution of trimethylamine. Does
the degree of ionization increase, decrease, or remain unchanged as the concentration
of (CH3)3N decreases? Give reasons for your answer.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
Questions


Arts, 21.09.2019 09:20



Mathematics, 21.09.2019 09:20


Mathematics, 21.09.2019 09:20


Biology, 21.09.2019 09:20


Spanish, 21.09.2019 09:20

History, 21.09.2019 09:20

Mathematics, 21.09.2019 09:20



Physics, 21.09.2019 09:20

Mathematics, 21.09.2019 09:20


History, 21.09.2019 09:20