Chemistry, 06.05.2020 02:10 jaejaeJae9534
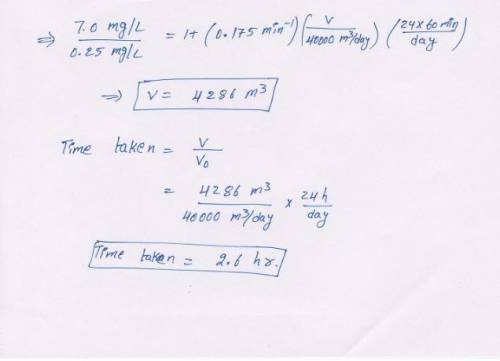
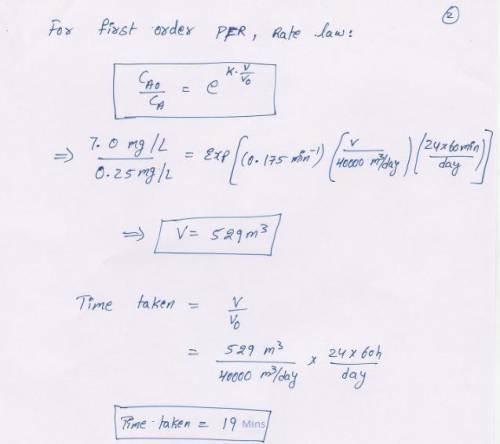
Water contains 7.0 mg/L of soluble ion (Fe2 ) that is to be oxidized by aeration to a concentration of 0.25 mg/L. The pH of the water is 6.0 and temperature is 12oC. Assume the dissolved oxygen in the water is in equilibrium with the surrounding atmosphere. Laboratory results indicate the pseudo first-order rate constant for oxygenation of Fe2 is 0.175/min. Assuming steady-state operations and a flow rate of 40,000 m3 /d, calculate the minimum detention time and reactor volume necessary for oxidation of Fe2 to Fe3 . Perform the calculations for both a CMFR and PFR. (You should be able to work this out from information provided in Chapters 3 and 4.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 04:40
Listen base your answer to the question on the information below.propane is a fuel that is sold in rigid, pressurized cylinders. most of the propane in a cylinder is liquid, with gas in the space above the liquid level. when propane is released from the cylinder, the propane leaves the cylinder as a gas. propane gas is used as a fuel by mixing it with oxygen in the air and igniting the mixture, as represented by the balanced equation below.c3h8(g) + 5o2(g) → 3co2(g) + 4h2o() + 2219.2 kja small amount of methanethiol, which has a distinct odor, is added to the propane to consumers detect a propane leak. in methanethiol, the odor is caused by the thiol functional group (–sh). methanethiol, ch3sh, has a structure that is very similar to the structure of methanol.what is the correct structural formula for a molecule of methanethiol
Answers: 3

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1
You know the right answer?
Water contains 7.0 mg/L of soluble ion (Fe2 ) that is to be oxidized by aeration to a concentration...
Questions





Health, 09.02.2021 14:00



Mathematics, 09.02.2021 14:00

Mathematics, 09.02.2021 14:00


Mathematics, 09.02.2021 14:00


English, 09.02.2021 14:00

History, 09.02.2021 14:00

Mathematics, 09.02.2021 14:00



Mathematics, 09.02.2021 14:00

Chemistry, 09.02.2021 14:00

History, 09.02.2021 14:00