
Chemistry, 06.05.2020 02:05 tylerbesson1
The addition of hydrochloric acid to a silver nitrate solution precipitates silver chloride according to the reaction:
AgNO3(aq) + HCl(aq) → AgCl(s) + HNO3(aq)
When 50.0 mL of 0.100 M AgNO3 is combined with 50.0 mL of 0.100 M HCl in a coffee-cup calorimeter, the temperature changes from 23.40 ∘C∘ to 24.21∘C.
Calculate Enthalpy chnage for the reaction as written. Use 1.00 g/mL as the density of the solution and Cs=4.18J/(g??C) as the specific heat capacity of the solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:10
What can be added to the examples section of each circle? endothermic: ice melting into water, and a heat pack becoming warm exothermic: a glow stick glowing, and fireworks exploding endothermic: ice melting into water, and an instant ice pack turning cold exothermic: fireworks exploding, and gasoline burning endothermic: a glow stick glowing, and a heat pack becoming warm exothermic: an instant ice pack turning cold, and ice melting into water endothermic: gasoline burning, and an instant ice pack turning cold exothermic: ice melting into water, and an instant ice pack turning cold
Answers: 1

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
The addition of hydrochloric acid to a silver nitrate solution precipitates silver chloride accordin...
Questions

Mathematics, 23.08.2021 16:10


Geography, 23.08.2021 16:10

Mathematics, 23.08.2021 16:10

Mathematics, 23.08.2021 16:10


Mathematics, 23.08.2021 16:10

Computers and Technology, 23.08.2021 16:10




Mathematics, 23.08.2021 16:10

Computers and Technology, 23.08.2021 16:10



Mathematics, 23.08.2021 16:10



World Languages, 23.08.2021 16:10

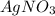 in 50.0 mL of 0.100 M of
in 50.0 mL of 0.100 M of 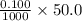 moles
moles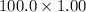 ) g = 100 g
) g = 100 g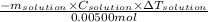
![\frac{-100g\times 4.18\frac{J}{g.^{0}\textrm{C}}\times [24.21-23.40]^{0}\textrm{C}}{0.00500mol}](/tpl/images/0645/0455/ac8b2.png)
 = change in temperature and negative sign is included as it is an exothermic reaction]
= change in temperature and negative sign is included as it is an exothermic reaction]


