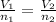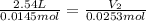Chemistry, 06.05.2020 02:01 leyslebravo2729
0.0145 moles of helium gas are introduced into a balloon so that the volume of the balloon is 2.54 liters. An additional amount of helium is added to the balloon so that the total amount of helium is 0.0253 moles. Calculate the final volume of the balloon.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
0.0145 moles of helium gas are introduced into a balloon so that the volume of the balloon is 2.54 l...
Questions

Mathematics, 03.11.2020 21:50

History, 03.11.2020 21:50

Mathematics, 03.11.2020 21:50

History, 03.11.2020 21:50

Mathematics, 03.11.2020 21:50


History, 03.11.2020 21:50

Mathematics, 03.11.2020 21:50


Biology, 03.11.2020 21:50

Mathematics, 03.11.2020 21:50

Mathematics, 03.11.2020 21:50

Chemistry, 03.11.2020 21:50


Mathematics, 03.11.2020 21:50

Social Studies, 03.11.2020 21:50


History, 03.11.2020 21:50


Mathematics, 03.11.2020 21:50