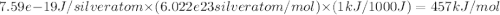The work function (ϕ) of a metal is the minimum energy needed to remove an electron from its surface. (a) Is it easier to remove an electron from a gaseous silver atom or from the surface of solid silver (ϕ = 7.59×10−19 J; IE = 731 kJ/mol)? (b) Explain the results in terms of the electron-sea model of metallic bonding.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1


Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
The work function (ϕ) of a metal is the minimum energy needed to remove an electron from its surface...
Questions

Mathematics, 09.11.2020 01:00

Geography, 09.11.2020 01:00

Physics, 09.11.2020 01:00



Mathematics, 09.11.2020 01:00






Spanish, 09.11.2020 01:00

Mathematics, 09.11.2020 01:00


English, 09.11.2020 01:00

Mathematics, 09.11.2020 01:00

Mathematics, 09.11.2020 01:00

English, 09.11.2020 01:00


Mathematics, 09.11.2020 01:00