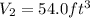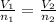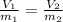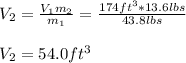Chemistry, 06.05.2020 03:13 davidpausiankhowv3nf
13.6 lbs of a gas occupies 174 ft3 at a certain pressure and temperature. The amount of gas is then increased to 43.8 lbs. What is the new volume for this amount assuming the pressure and temperature haven’t changed? Answer in units of ft .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
You know the right answer?
13.6 lbs of a gas occupies 174 ft3 at a certain pressure and temperature. The amount of gas is then...
Questions


Mathematics, 22.10.2019 21:00

Business, 22.10.2019 21:00



Computers and Technology, 22.10.2019 21:00











Computers and Technology, 22.10.2019 21:00