
Chemistry, 06.05.2020 03:11 acavalieri72
Predict whether ΔS for each reaction would be greater than zero, less than zero, or too close to zero to decide.
ΔS > 0; ΔS < 0; too close to decide
I2(g) + Cl2(g) > 2ICl(g)
2NOBr(g) > 2NO(g) + Br2(g)
CO2(g) + H2(g) > CO(g) + H2O(g)
2H2O2(I) > 2H2O(I) + O2(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Predict whether ΔS for each reaction would be greater than zero, less than zero, or too close to zer...
Questions



Mathematics, 14.02.2022 01:00



Mathematics, 14.02.2022 01:00

English, 14.02.2022 01:00

Mathematics, 14.02.2022 01:00

Mathematics, 14.02.2022 01:00

Physics, 14.02.2022 01:00

Mathematics, 14.02.2022 01:00

Mathematics, 14.02.2022 01:00

Mathematics, 14.02.2022 01:00


Mathematics, 14.02.2022 01:00



Chemistry, 14.02.2022 01:00

English, 14.02.2022 01:00

Mathematics, 14.02.2022 01:00

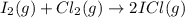 : too close to decide.
: too close to decide.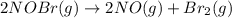 :
: 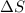 > 0.
> 0.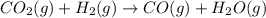 : too close to decide.
: too close to decide.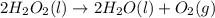 :
: 

