
Chemistry, 06.05.2020 04:33 bobbyhsu3751
Suppose a 500.mL flask is filled with 1.9mol of NO3 and 1.6mol of NO. The following reaction becomes possible: NO3gNOg 2NO2g The equilibrium constant K for this reaction is 8.33 at the temperature of the flask. Calculate the equilibrium molarity of NO. Round your answer to two decimal places

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1
You know the right answer?
Suppose a 500.mL flask is filled with 1.9mol of NO3 and 1.6mol of NO. The following reaction becomes...
Questions

Biology, 12.12.2020 16:30

Social Studies, 12.12.2020 16:30


Mathematics, 12.12.2020 16:30

English, 12.12.2020 16:30

Physics, 12.12.2020 16:30

Social Studies, 12.12.2020 16:30

Mathematics, 12.12.2020 16:30



Mathematics, 12.12.2020 16:30


History, 12.12.2020 16:30

Chemistry, 12.12.2020 16:30


Social Studies, 12.12.2020 16:30




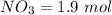
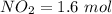
![[NO_3] = \frac{number \ of \ moles}{volume}](/tpl/images/0646/3223/b324f.png)
![[NO_3] = \frac{1.9}{0.500}](/tpl/images/0646/3223/e2e7e.png)
![[NO_3] = 3.8 \ M](/tpl/images/0646/3223/8a5ca.png)
![[NO_2] = \frac{number \ of \ moles}{volume}](/tpl/images/0646/3223/1e0e1.png)
![[NO_2] = \frac{}{} \frac{1.6}{0.500}](/tpl/images/0646/3223/24dfd.png)
![[NO_2] = 3.2 \ M](/tpl/images/0646/3223/096c8.png)
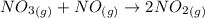
![K_c=\frac{[NO_2]^2}{[NO_3][NO]}](/tpl/images/0646/3223/498af.png)
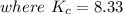
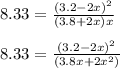
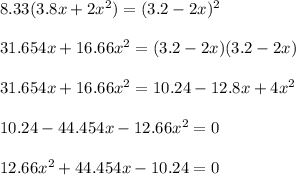
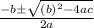
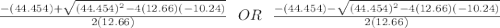
![[NO_3] = 3.8 +x = 3.8 + 0.21695](/tpl/images/0646/3223/7da74.png)
![[NO_2] = 3.2 +x = 3.2 + 0.21695](/tpl/images/0646/3223/43cc9.png)


