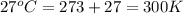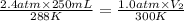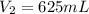Chemistry, 06.05.2020 04:32 patriciahonsakpa6u5f
A diver exhales a bubble with a volume of 250 mL at a pressure of 2.4 atm and a temperature of 15 °C. What is the volume of the bubble when it reaches the surface where the pressure is 1.0 atm and the temperature is 27 °C, if the moles are constant?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2


Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
A diver exhales a bubble with a volume of 250 mL at a pressure of 2.4 atm and a temperature of 15 °C...
Questions


English, 05.12.2020 01:10

History, 05.12.2020 01:10


Business, 05.12.2020 01:10

Mathematics, 05.12.2020 01:10

Chemistry, 05.12.2020 01:10


Mathematics, 05.12.2020 01:10


Mathematics, 05.12.2020 01:10

History, 05.12.2020 01:10

Mathematics, 05.12.2020 01:10

Mathematics, 05.12.2020 01:10





Mathematics, 05.12.2020 01:10

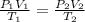
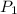 = initial pressure of gas = 2.4 atm
= initial pressure of gas = 2.4 atm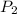 = final pressure of gas = 1.0 atm
= final pressure of gas = 1.0 atm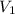 = initial volume of gas = 250 mL
= initial volume of gas = 250 mL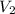 = final volume of gas = ?
= final volume of gas = ?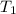 = initial temperature of gas =
= initial temperature of gas = 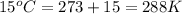
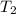 = final temperature of gas =
= final temperature of gas =