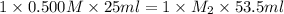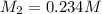Chemistry, 05.02.2020 08:02 sascsl2743
In a titration, 25.0 ml of 0.500 m khp is titrated to the equivalence point with naoh. the final solution volume is 53.5 ml. what is the value of m2 for the reaction?
0.439 m
0.500 m
0.234 m
1.07 m

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
In a titration, 25.0 ml of 0.500 m khp is titrated to the equivalence point with naoh. the final sol...
Questions

Social Studies, 06.10.2019 18:00

Social Studies, 06.10.2019 18:00


Health, 06.10.2019 18:00









History, 06.10.2019 18:00


History, 06.10.2019 18:00

Mathematics, 06.10.2019 18:00


Health, 06.10.2019 18:00

Social Studies, 06.10.2019 18:00

Mathematics, 06.10.2019 18:00

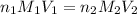
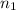 = basicity of an acid = 1
= basicity of an acid = 1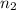 = acidity of a base = 1
= acidity of a base = 1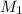 = concentration of KHP = 0.500 M
= concentration of KHP = 0.500 M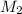 = concentration of NaOH = ?
= concentration of NaOH = ?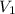 = volume of KHP = 25 ml
= volume of KHP = 25 ml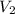 = volume of NaOH = 53.5 ml
= volume of NaOH = 53.5 ml