
Chemistry, 06.05.2020 05:15 ayoismeisalex
A solid weak acid is weighed, dissolved in water and diluted to exactly 50.00 ml. 25.00 ml of the solution is taken out and is titrated to a neutral endpoint with 0.10 m NaOH. The titrated portion is then mixed with the remaining untitrated portion and the pH of the mixture is measured.
Mass of acid weighed out (grams) 0.755
Volume of NaOH required to reach endpoint: (ml) 18.8
pH of the mixture (half neutralized solution) 3.51
1. What is the pKa of the acid?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1


Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
You know the right answer?
A solid weak acid is weighed, dissolved in water and diluted to exactly 50.00 ml. 25.00 ml of the so...
Questions

Chemistry, 29.08.2019 22:30

Mathematics, 29.08.2019 22:30




English, 29.08.2019 22:30

Chemistry, 29.08.2019 22:30

Computers and Technology, 29.08.2019 22:30




Computers and Technology, 29.08.2019 22:30

Biology, 29.08.2019 22:30


Engineering, 29.08.2019 22:30






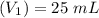

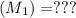
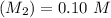

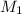 the subject of the formula; we have:
the subject of the formula; we have:
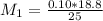
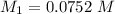
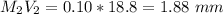
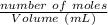
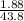
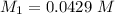
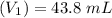
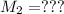
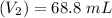
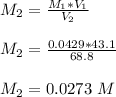

![pH = pKa + log \frac{[salt]}{[acid]}](/tpl/images/0646/6370/2dc0b.png)
![3.51= pKa + log \frac{[0.0273]}{[0.0273]} \\ \\ 3.51= pKa + log \ 1 \\ \\ 3.51= pKa + 0 \\ \\ pKa = 3.51](/tpl/images/0646/6370/56a03.png)



