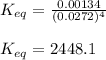Chemistry, 06.05.2020 06:36 papasully1
Nickel and carbon monoxide react to form nickel carbonyl, like this: (s)(g)(g) At a certain temperature, a chemist finds that a reaction vessel containing a mixture of nickel, carbon monoxide, and nickel carbonyl at equilibrium has the following composition: compound amount
Calculate the value of the equilibrium constant

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2

You know the right answer?
Nickel and carbon monoxide react to form nickel carbonyl, like this: (s)(g)(g) At a certain temperat...
Questions



Mathematics, 26.12.2021 14:00


English, 26.12.2021 14:00

Physics, 26.12.2021 14:00



Social Studies, 26.12.2021 14:00


Geography, 26.12.2021 14:00


History, 26.12.2021 14:00

Geography, 26.12.2021 14:00

Social Studies, 26.12.2021 14:00

Spanish, 26.12.2021 14:00


Social Studies, 26.12.2021 14:00

Mathematics, 26.12.2021 14:00

Business, 26.12.2021 14:00

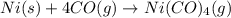
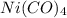 0.597 g
0.597 g
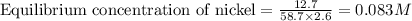
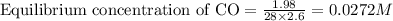
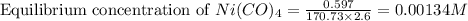
![K_{eq}=\frac{[Ni(CO)_4]}{[CO]^4}](/tpl/images/0647/3467/b75d8.png)