
Chemistry, 06.05.2020 07:41 punkinrichard1oxon2i
Suppose 0.829g of zinc chloride is dissolved in 100.mL of a 0.60M aqueous solution of potassium carbonate. Calculate the final molarity of chloride anion in the solution. You can assume the volume of the solution doesn't change when the zinc chloride is dissolved in it. Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
Suppose 0.829g of zinc chloride is dissolved in 100.mL of a 0.60M aqueous solution of potassium carb...
Questions

Mathematics, 29.03.2021 03:20


Mathematics, 29.03.2021 03:20


Mathematics, 29.03.2021 03:20

Mathematics, 29.03.2021 03:20

Mathematics, 29.03.2021 03:30


Chemistry, 29.03.2021 03:30


Mathematics, 29.03.2021 03:30

Chemistry, 29.03.2021 03:30



Mathematics, 29.03.2021 03:30




History, 29.03.2021 03:30

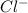 anion in solution is 0.122 M.
anion in solution is 0.122 M.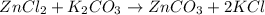
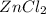 .
.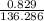 moles of
moles of 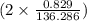 moles
moles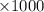
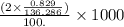 M
M

