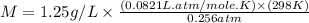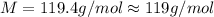Chemistry, 06.05.2020 07:30 Epicgible8136
Chloroform is a volatile liquid once commonly used in the laboratory but now being phased out due to its ozone depletion potential. If the pressure of gaseous chloroform in a flask is 195 mm Hg at 25°C and its density is 1.25 g/L, what is the molar mass of chloroform? A) 10.0 g/mol B) 76.3 g/mol C) 119 g/mol D) None of these

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1
You know the right answer?
Chloroform is a volatile liquid once commonly used in the laboratory but now being phased out due to...
Questions

Advanced Placement (AP), 13.10.2020 05:01


Chemistry, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01

History, 13.10.2020 05:01

Health, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01




Geography, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01





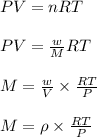
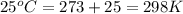
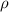 = density of gas = 1.25 g/L
= density of gas = 1.25 g/L