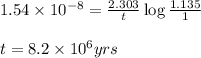Chemistry, 06.05.2020 07:22 babyduckies37
The contents of a rock have a 206Pb to 238U mass ratio of 0.135:1.00. Assuming that the rock did not contain any 206Pb at the time of its formation, determine the age of the rock. Uranium-238 decays to lead-206 with a half-life of 4.5 billion years. Express the time to two significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

You know the right answer?
The contents of a rock have a 206Pb to 238U mass ratio of 0.135:1.00. Assuming that the rock did not...
Questions





Business, 12.07.2019 00:00



Business, 12.07.2019 00:00












English, 12.07.2019 00:00

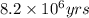
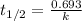
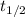 = half life of the reaction = 4.5 billion years =
= half life of the reaction = 4.5 billion years = 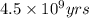
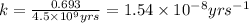
![k=\frac{2.303}{t}\log\frac{[A_o]}{[A]}](/tpl/images/0647/7147/f1041.png)
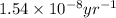
![[A_o]](/tpl/images/0647/7147/dc622.png) = initial amount of the sample = [1.00 + 0.135] = 1.135 g
= initial amount of the sample = [1.00 + 0.135] = 1.135 g