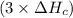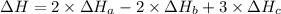2 Fe(s) + 6 HCl(aq) →2 FeCl3(aq) + 3 H2(g) ΔHa Fe2O3(s) + 6 HCl(aq) → 2 FeCI3(aq) + 3 H2O(l) ΔHb 2 H2(g) + O2(g) → 2 H2O(l) ΔHc Show how these equations must be summed together according to Hess's Law to determine ΔH for the combustion of iron (target equation shown below). Also show clearly how the ΔH values of each of the three reactions must be manipulated to determine the enthalpy of combustion of iron. 4 Fe(s) + 3 O2(g) → 2 Fe2O3(s) ΔH = ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1

Chemistry, 23.06.2019 08:00
At 35.0°c and 3.00 atm pressure, a gas has a volume of 1.40 l. what pressure does the gas have at 0.00°c and a volume of 0.950 l? which equation should you use? p2= p1v1t2/t1v2what is the pressure of the gas? 3.92 atm these are the answers
Answers: 1
You know the right answer?
2 Fe(s) + 6 HCl(aq) →2 FeCl3(aq) + 3 H2(g) ΔHa Fe2O3(s) + 6 HCl(aq) → 2 FeCI3(aq) + 3 H2O(l) ΔHb 2 H...
Questions


Mathematics, 24.04.2021 02:40

Mathematics, 24.04.2021 02:40



Mathematics, 24.04.2021 02:40



Health, 24.04.2021 02:40

History, 24.04.2021 02:40



Mathematics, 24.04.2021 02:40

Mathematics, 24.04.2021 02:40


Health, 24.04.2021 02:40

English, 24.04.2021 02:40

Social Studies, 24.04.2021 02:40


History, 24.04.2021 02:40

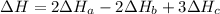
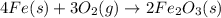
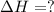
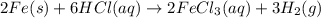 ;
; 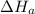
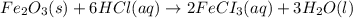 ;
; 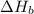
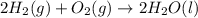 ;
; 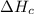
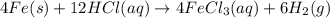 ;
; 
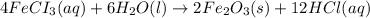 ;
; 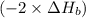
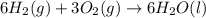 ;
;