
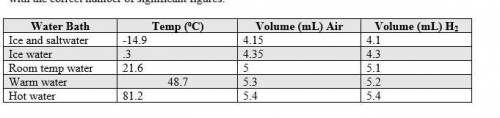
1. The actual value for absolute zero in degrees Celsius is −273.15. Use the formula below to determine your percent error for both gas samples.
|experimental value – actual value| x 100
actual value
2. If the atmospheric pressure in the laboratory is 1.2 atm, how many moles of gas were in each syringe? (Hint: Choose one volume and temperature pair from your data table to use in your ideal gas law calculation.)
Conclusion:
Write a conclusion statement that addresses the following questions:
• How did your experimental absolute zero value compare to the accepted value?
• Does your data support or fail to support your hypothesis (include examples)?
• Discuss any possible sources of error that could have impacted the results of this lab.
• How do you think the investigation can be explored further?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2



Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
1. The actual value for absolute zero in degrees Celsius is −273.15. Use the formula below to determ...
Questions

English, 06.04.2020 18:25





History, 06.04.2020 18:25



Social Studies, 06.04.2020 18:25



Mathematics, 06.04.2020 18:25

Mathematics, 06.04.2020 18:25

Mathematics, 06.04.2020 18:25



Mathematics, 06.04.2020 18:26


Physics, 06.04.2020 18:26



