Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
You know the right answer?
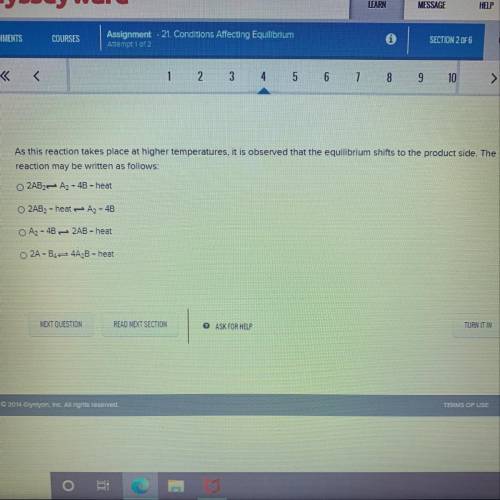
As this reaction takes place at higher temperatures, it is observed that the equilibrium shifts to t...
Questions


Mathematics, 28.04.2021 21:50



Mathematics, 28.04.2021 21:50

Mathematics, 28.04.2021 21:50

Biology, 28.04.2021 21:50

Engineering, 28.04.2021 21:50

Chemistry, 28.04.2021 21:50


Physics, 28.04.2021 21:50




Mathematics, 28.04.2021 21:50



Mathematics, 28.04.2021 21:50

Mathematics, 28.04.2021 21:50