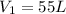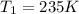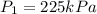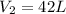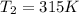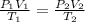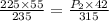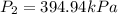Chemistry, 26.04.2020 03:48 zirconium16
A 55.0 L sample of gas at 235 K exerts a pressure of 225 KPa. If its volume is decreased to 42.0 L and the temperature rises to 315 K, what is its new pressure?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:40
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2


Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
A 55.0 L sample of gas at 235 K exerts a pressure of 225 KPa. If its volume is decreased to 42.0 L a...
Questions

Advanced Placement (AP), 28.10.2020 04:30


Biology, 28.10.2020 04:30

English, 28.10.2020 04:30

Biology, 28.10.2020 04:30

Biology, 28.10.2020 04:30


History, 28.10.2020 04:30


Biology, 28.10.2020 04:30




English, 28.10.2020 04:30

Mathematics, 28.10.2020 04:30

History, 28.10.2020 04:30


English, 28.10.2020 04:30