Chemistry, 25.04.2020 23:17 WonTonBagel
A helium sample occupies 1.50 L of space at 124.6 kPa. What pressure would the helium need to experience to have a volume of 0.75 L?
A. 130 kPa
B. 97 kPa
C. 250 kPa
D. 62 kPa

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
A helium sample occupies 1.50 L of space at 124.6 kPa. What pressure would the helium need to experi...
Questions




Social Studies, 26.09.2019 08:50


Mathematics, 26.09.2019 08:50

English, 26.09.2019 08:50

Social Studies, 26.09.2019 08:50

Biology, 26.09.2019 08:50

History, 26.09.2019 08:50


Social Studies, 26.09.2019 08:50




Mathematics, 26.09.2019 08:50


Business, 26.09.2019 08:50

Biology, 26.09.2019 08:50

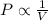
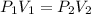
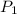 = initial pressure = 124.6 kPa
= initial pressure = 124.6 kPa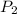 = final pressure = ?
= final pressure = ?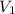 = initial volume = 1.50 L
= initial volume = 1.50 L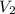 = final volume = 0.75 L
= final volume = 0.75 L