Chemistry, 25.04.2020 04:49 jessieeverett432
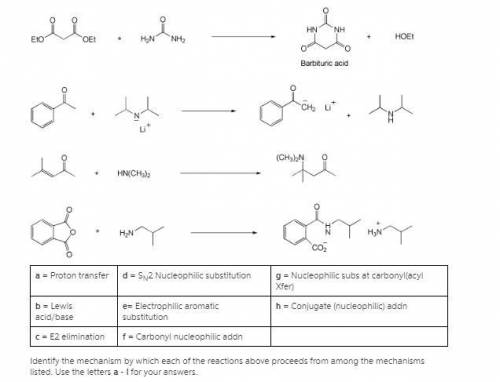
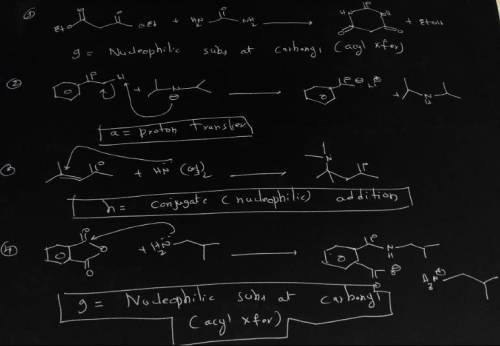
A = Proton transfer d = SN2 Nucleophilic substitution g = Nucleophilic subs at carbonyl(acyl Xfer) b = Lewis acid/base e= Electrophilic aromatic substitution h = Conjugate (nucleophilic) addn c = E2 elimination f = Carbonyl nucleophilic addn Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. n(nh3)3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
What is the relationship between wind and ocean waves? question 17 options: wind moving at higher speeds will transfer more energy to the water, resulting in stronger waves. wind moving at higher speeds will transfer energy over a larger part of the ocean water, resulting in waves with a shorter wavelength. winds moving at higher speeds with cause water to move forward at faster rates, causing larger ocean waves. winds moving at higher speeds will affect deeper water, resulting in waves that move at a faster rate. how do temperature and salinity affect deepwater currents? question 15 options: as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 06:00
What volume of 0.500 mol/l hydrochloric acid, hci (aq) is required to react completely with 1.00 g of aluminum hydroxide, ai(oh)3 (s)?
Answers: 1
You know the right answer?
A = Proton transfer d = SN2 Nucleophilic substitution g = Nucleophilic subs at carbonyl(acyl Xfer) b...
Questions

Social Studies, 10.11.2020 19:00

Biology, 10.11.2020 19:00


Mathematics, 10.11.2020 19:00

History, 10.11.2020 19:00


Mathematics, 10.11.2020 19:00


Mathematics, 10.11.2020 19:00

Mathematics, 10.11.2020 19:00






Mathematics, 10.11.2020 19:00

Mathematics, 10.11.2020 19:00

Mathematics, 10.11.2020 19:00


English, 10.11.2020 19:00