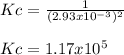Titanium and chlorine react to form titanium(IV) chloride, like this:
TiCl3 → Ti(s)+ 2Cl2(g)<...

Chemistry, 25.04.2020 04:26 rachelbrooks764
Titanium and chlorine react to form titanium(IV) chloride, like this:
TiCl3 → Ti(s)+ 2Cl2(g)
At a certain temperature, a chemist finds that a 5.2L reaction vessel containing a mixture of titanium, chlorine, and titanium(IV) chloride at equilibrium has the following composition:
Compound Amount
TiCl4 4.18g
Ti 1.32g
Cl2 1.08g
Calculate the value of the equilibrium constant Kc for this reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
Questions



Arts, 09.12.2021 07:40

Social Studies, 09.12.2021 07:40


Chemistry, 09.12.2021 07:40


Mathematics, 09.12.2021 07:40


English, 09.12.2021 07:40



Mathematics, 09.12.2021 07:40

Mathematics, 09.12.2021 07:40

Business, 09.12.2021 07:40


Mathematics, 09.12.2021 07:40

Mathematics, 09.12.2021 07:40

Advanced Placement (AP), 09.12.2021 07:40

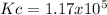
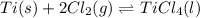
![Kc=\frac{1}{[Cl_2]^2}](/tpl/images/0626/7109/175a7.png)
![[Cl_2]_{eq}=\frac{1.08gCl_2*\frac{1molCl_2}{70.9gCl_2}}{5.2L}=2.93x10^{-3}M](/tpl/images/0626/7109/9b00a.png)