
Chemistry, 08.10.2019 18:30 torresalysabeth
In a titration, 25.0 ml of 0.500 m khp is titrated to the equivalence point with naoh. the final solution volume is 53.5 ml. what is the value of v2 for the reaction?
a: 25
b; 0.5
c: 53.5
d: 28.5

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
In a titration, 25.0 ml of 0.500 m khp is titrated to the equivalence point with naoh. the final sol...
Questions

Mathematics, 11.12.2019 08:31


Mathematics, 11.12.2019 08:31

Mathematics, 11.12.2019 08:31

English, 11.12.2019 08:31




Mathematics, 11.12.2019 08:31

Mathematics, 11.12.2019 08:31

Mathematics, 11.12.2019 08:31


Biology, 11.12.2019 08:31



English, 11.12.2019 08:31



Biology, 11.12.2019 08:31

English, 11.12.2019 08:31

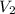 for the reaction is, (A) 28.5 ml
for the reaction is, (A) 28.5 ml

