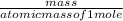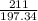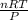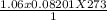1. Zinc metal reacts with 100 mL of 6.00 M H2SO4. How many grams of Zinc Sulfate are produced and how many liters of Hydrogen gas would be released?
2.A 211 g sample of BaCO3 is reacted with HNO3. Assuming the acid is present is excess, what mass and volume of CO2 gas will be produced?

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2

Chemistry, 23.06.2019 06:40
1.) which of the following is a molecule but not a compound? a.he b.f2 c.h2o d.ch4 2.) what is a physical combination of substances? a.a compound b.a molecule c.a mixture d.an element 3.) what is a chemical combination of substances? a.a compound b.an atom c.a mixture d.an element 4.) what is the relationship between the solute and solvent in a solution? a.they form a compound b.they form a mixture c.they form molecules d.they form chemical bonds 5.) the gases in air dissolve in water. what would be one way to reduce the amount of a gas dissolved in water? a.add more water b.reduce the air pressure c.increase the air pressure d.stir the water 6.) how would you determine the solubility of a substance? a.find how well it dissolved various substances. b.find the mass and the volume of the substance. c.find the temperature at which the substance evaporated. d.find how much i was able to dissolve in a solute. 7.) the periodic table organizes all of the kinds of a.molecules. b.compounds. c.atoms. d.ions. 8.)what distinguishes two substances combined to become a compound vs. two substances combined to become a mixture? a.whether they can be easily separated b.whether they chemically bond together c.whether they both are visible d.whether they are heterogeneous 9.) the principle components of air are: n2 78% o2 21% ar 0.95% co2 0.038% this is a solution of a.molecules and atoms. b.molecules. c.compounds and molecules. d.atoms.
Answers: 1

Chemistry, 23.06.2019 08:00
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1
You know the right answer?
1. Zinc metal reacts with 100 mL of 6.00 M H2SO4. How many grams of Zinc Sulfate are produced and ho...
Questions




Mathematics, 07.12.2021 04:10

Business, 07.12.2021 04:10

Mathematics, 07.12.2021 04:10

Health, 07.12.2021 04:10




Mathematics, 07.12.2021 04:10

Chemistry, 07.12.2021 04:20

Mathematics, 07.12.2021 04:20



Mathematics, 07.12.2021 04:20

Mathematics, 07.12.2021 04:20


Mathematics, 07.12.2021 04:20

Mathematics, 07.12.2021 04:20

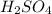 ⇒ ZnS
⇒ ZnS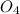 +
+ 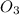 + 2HN
+ 2HN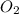 +
+ 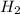 O
O