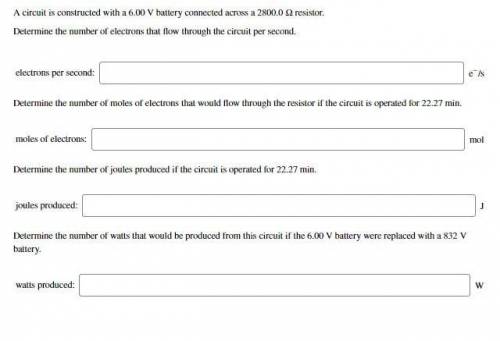
A circuit is constructed with a 6.00 V battery connected across a 2800.0 Ω resistor.
a.)...

A circuit is constructed with a 6.00 V battery connected across a 2800.0 Ω resistor.
a.) Determine the number of electrons that flow through the circuit per second. (answer in e^-/s)
b.) Determine the number of moles of electrons that would flow through the resistor if the circuit is operated for 22.27 min. (answer in moles)
c.) Determine the number of joules produced if the circuit is operated for 22.27 min. (answer in joules (J))
d.) Determine the number of watts that would be produced from this circuit if the 6.00 V battery were replaced with a 832 V battery. (answer in watts (W))
This is an analytical chemistry problem.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
In any energy conversion, some of the energy is lost to the environment as question 5 options: electrical energy potential energy sound energy thermal energy
Answers: 1

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1
You know the right answer?
Questions


Mathematics, 01.06.2020 02:58




Mathematics, 01.06.2020 02:58

Physics, 01.06.2020 02:58

English, 01.06.2020 02:58

History, 01.06.2020 02:58

Mathematics, 01.06.2020 02:58

English, 01.06.2020 02:58

English, 01.06.2020 02:58



Mathematics, 01.06.2020 02:58


World Languages, 01.06.2020 02:58

Mathematics, 01.06.2020 02:58




