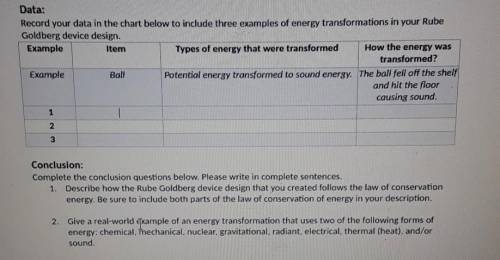
Data:
Record your data in the chart below to include three examples of energy transformations i...

Data:
Record your data in the chart below to include three examples of energy transformations in your Rube
Goldberg device design.
Example
Item
Types of energy that were transformed How the energy was
transformed?
Example
Ball Potential energy transformed to sound energy. The ball fell off the shelf
and hit the floor
causing sound.
Conclusion:
Complete the conclusion questions below. Please write in complete sentences.
1. Describe how the Rube Goldberg device design that you created follows the law of conservation
energy. Be sure to include both parts of the law of conservation of energy in your description.
2. Give a real-world examplepf an energy transformation that uses two of the following forms of energy: chemical, mechanitual, nuclear, gravitational, radiant, electrical, thermal (heat), and/or
sound.
IF YOU HELP I WILL MARK BRAINLIEST! SO PLEASEE

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
Questions




History, 26.09.2019 00:30

History, 26.09.2019 00:30

Biology, 26.09.2019 00:30


Mathematics, 26.09.2019 00:30


Social Studies, 26.09.2019 00:30

Health, 26.09.2019 00:30

Mathematics, 26.09.2019 00:30

History, 26.09.2019 00:30


Mathematics, 26.09.2019 00:30

English, 26.09.2019 00:30

History, 26.09.2019 00:30


History, 26.09.2019 00:30

Mathematics, 26.09.2019 00:30



