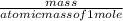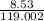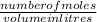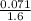Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1
You know the right answer?
Calculate the molarity of 1.60 L of a solution containing 8.53 g of dissolved KBr.
HINT: Calcu...
HINT: Calcu...
Questions





English, 25.07.2019 00:30



Mathematics, 25.07.2019 00:30

History, 25.07.2019 00:30

Mathematics, 25.07.2019 00:30



English, 25.07.2019 00:30






Mathematics, 25.07.2019 00:30