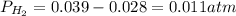Chemistry, 24.04.2020 18:10 kipper5760
You collect a sample of hydrogen gas in an inverted buret by displacement of water at 24.8oC. The water level in the buret was 15.89 cm above the water level in the water bath. The volume of the gas in the buret was determined to be 32.20 mL. If the current atmospheric pressure was 29.68" of Hg, what is the pressure of the dry hydrogen in the buret in atm

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

You know the right answer?
You collect a sample of hydrogen gas in an inverted buret by displacement of water at 24.8oC. The wa...
Questions

French, 20.09.2020 05:01



Business, 20.09.2020 05:01


Mathematics, 20.09.2020 05:01


Mathematics, 20.09.2020 05:01


Mathematics, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01

Computers and Technology, 20.09.2020 05:01

World Languages, 20.09.2020 05:01


Physics, 20.09.2020 05:01

History, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01

Advanced Placement (AP), 20.09.2020 05:01

Physics, 20.09.2020 05:01