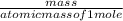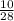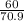Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2


Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3
You know the right answer?
Si + 2Cl2 + SiC14
What mass of SiCl4 is formed when 10.0 grams of Si and 60.0
grams of C...
What mass of SiCl4 is formed when 10.0 grams of Si and 60.0
grams of C...
Questions

Mathematics, 30.07.2019 06:30


Mathematics, 30.07.2019 06:30


History, 30.07.2019 06:30




Mathematics, 30.07.2019 06:40



Computers and Technology, 30.07.2019 06:40


Social Studies, 30.07.2019 06:40



Geography, 30.07.2019 06:40



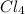 = 169.8 grams/mole
= 169.8 grams/mole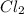 ⇒ Si
⇒ Si